How ‘special’ are bats as reservoirs of human disease?
In recent years, bats have also become known as
the source taxa—scientifically called ‘reservoir hosts’—for the viruses that cause rabies, Ebola, Nipah, SARS—and most recently,
COVID-19. Is it accurate to portray bats as high risk sources of human disease? Several meta-analyses summarizing research on pathogens that jump from animals to humans, known as zoonoses, have suggested that, yes—on a per species metric—bats do indeed host more viruses that infect people than do other mammals (e.g.
Olival et al. 2017,
Luis et al. 2013). A
new study, however, suggests that this accusation may be unfair.
 |
Examples of natural reservoir hosts from three orders with high species diversity. From left to right: Light-vented bulbul (Pycnonotus sinenesis), bank vole (Myodes glareolus), and intermediate horseshoe bat (Rhinolophus affinis). Photos by Arthur Chapman, Frank Vassen, and Pipat Soisook, respectively.
|
In their study, Mollentze and Streicker analyzed 429 associations between viruses and animal hosts to examine whether some taxa are more likely to host human-infecting viruses than others. The authors expanded the scope of prior work to consider bird hosts of zoonotic viruses in addition to mammals, while simultaneously applying more stringent criteria for inclusion in their database than have previous efforts—focusing only on strict reservoir hosts, eliminating taxa with fewer than five viruses represented, and summarizing interactions by host taxonomic order. For example, rabies can be sourced from multiple bat species, but Mollentze and Streicker only associated the virus with the order Chiroptera once, so as to avoid overrepresentation of the same data in the pipeline.
Many previous studies have suggested that those species which most closely resemble humans based on their DNA may be more likely to source human-infecting viruses. Mollentze and Streicker, in contrast, found no effect of host order or phylogeny on the probability of a virus spilling over into the human population. These differing conclusions may result from differences in the databases queried or differences in the methods used to assess the genetic relatedness of the viral hosts examined. As a result of their findings, Mollentze and Streicker eschew the idea of “special reservoirs” for human diseases to instead emphasize a ‘reservoir richness hypothesis’ – meaning host taxa with many species (like bats and rodents, which together make up 60% of all mammals) tend to host more viruses in total within the order. The proportion of these viruses which are zoonotic appears to be relatively well conserved across taxa, meaning that bats host many zoonoses simply because there are many different species of bats, which as a group, host many viruses. By this account, bats do source human viruses – but they are no different in this respect to any other mammal or bird!
 |
|
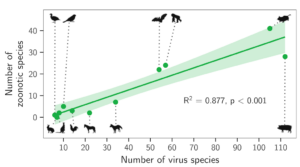
Source: Mollentze and Streicker (2020) Figure 3. Relationship between the number of virus species and the number of zoonotic species maintained by each reservoir group. The line above shows linear regression fit, with its 95% CI indicated by the shading. Both the total number of viruses associated with each reservoir group and the number of zoonoses matched expectations under the reservoir richness hypothesis. The total number of viruses associated with bats is consistent with expectations, not exceptionally higher.
|
In addressing the role of bats as hosts for zoonotic viruses, Mollentze and Streicker consider only the probability of a bat-borne virus infecting a human. The authors do not address a zoonotic virus’s capacity to cause harm to a human host (called ‘virulence’) or the likelihood that a bat-borne virus will adapt to effectively transmit human-to-human (like COVID-19) with no need for future cross species’ jumps to reseed an epidemic. For example,
our work has previously demonstrated that bat-borne viruses cause higher case fatality rates—higher virulence—in human hosts than viruses derived from other mammals. We hypothesize that this virulence in humans could result from
faster viral spread rates evolving in bat-borne viruses due to their unique
tolerance for viral infection thought to have evolved as a consequence of their
adaptations related to flight. Thus, it may be the
type of viruses, not the quantity, that makes bats unique. Future work is needed to address this hypothesis further.
Doubtless, scientists will continue to debate – as scientists like to do – whether certain taxa should be portrayed as ‘special reservoirs’ of emerging zoonoses. At present, the waters are murky on this front, but for us, the message is the same: Bats are special, no matter what, for the many services—pollination, pest control, fertilizer generation—that they provide to our ecosystems! Nonetheless, some bat species do host viruses that can cause human disease—but so do most animals. We are only beginning to understand the full scope of pathogens hosted by animal reservoirs, and only through continued study of bat-borne viruses—and viruses derived from many wild species—will we be better prepared to face the next pandemic. Pathogens tend to spill over
when intact ecosystems are threatened and humans are brought into contact with species they might not otherwise encounter. By preserving bat habitats and safeguarding bat livelihoods, we can
protect both wildlife conservation and human public health alike!



